(Bild: Crosssection: breakdown structure of a HBr RFB cell with different sealing concepts (Fig.: IMA))
30.10.2019 Sealing in redox flow batteries
Influence of HBr chemical impact
This research project determines what the chemical impact is of HBr-Br2 electrolyte on elastomer seals in redox flow batteries (RFBs). Proper energy storage is the solution to promote electricity from green energy. Hydrogen bromine redox flow batteries are a new generation of RFBs, which are presently in fast development. Successful application of sealing technology in such designs even benefit other accompanying RFBs. The primary task is to select proper rubber materials, which are chemical and mechanical stable. Fluorinated propylene monomer (FPM) and ethylene propylene diene monomer (EPDM) rubbers deem to be good suitors.
State of art of RFBs
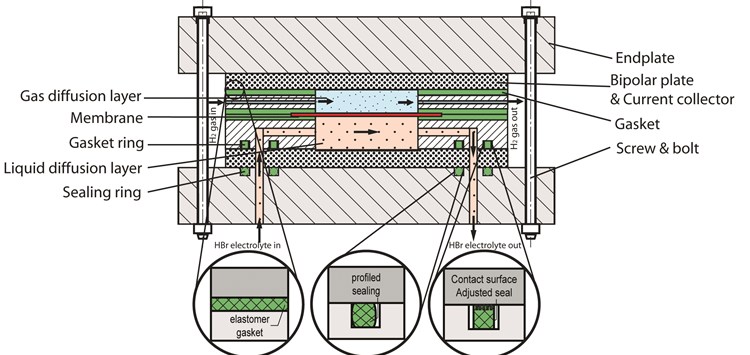
A redox flow battery (RFB) is like any other kind of battery, a way to store energy. In this case electrochemical energy which can be transformed into electrical energy. A noticeable difference with conventional batteries: the electrodes in RFBs do not take part in the redox reaction. The design structure generally exists of the components as shown in figure 1. The electrodes have the function of conducting and distributing electrons. The potential energy is stored in the electrolyte, which is separated into external negative and positive containers. A benefit of RFBs is the possibility of decoupling energy and power (by changing sizes). Currently RFBs are more expensive compared to other energy storage batteries due to the need of pumps and expensive materials, but RFBs have the longest life cycle expectancy so over time they can be more economic.
In a hydrogen bromine RFB, one container holds aqueous HBr-Br2 while the other container holds H2-gas. In the cell, the gas side is the gas diffusion layer (GDL) where H2 gas flows through. At the other side of the membrane is the liquid diffusion layer (LDL) where aqueous HBr and Br flows. The ratio HBr-Br depends on the state of charge of the battery. Figure 2 reveals one way of the battery, charging or discharging depends on the polarity of the battery. The chemical reaction also goes the other way. A proton exchange membrane (PEM) separates the two fluids in the battery cell. A common composite material for these RFB membranes exists of woven reinforcing polyether ether ketone (PEEK) covered in a resin of perfluoro sulfonic acid (PFSA). This is a critical component, which needs to be taken into account when leakage occurs. Leakage also happens because of crossover through the membrane, but this phenomenon is not in the scope of this research.The focus lays on elastomer gaskets and seals, which are placed between the membrane and electrodes and between the flow frame and bipolar plate. Sealing rings are mounted at the inlet and outlets of the endplates, flow frames and pumps. It is critical that at all these locations leakage remains minimal. The meaning of compatibility has different answers. Depending on the application, a seal has to withstand all environmental effects. Alternatively, in such a way that it is acceptable for the application to function well for a certain timespan. Realise that zero leakage is not possible, for example there will always be leakage of H2-gas due to permeability of materials. The chemical compatibility of the seal is in correlation to the mechanical properties. The electrolyte has influence on the seal, which may be acceptable as long as it does not shorten the lifespan of the redox flow battery. It is of impeccable importance that the seal remains functional in this application. Adequate leakage of H2-gas near a heat source is flammable. Evaporated bromine and H2-gas are poisonous. Although bromine has a very distinct salty smell, but H2-gas is odourless. Leakage of the acid electrolyte creates damage to the whole RFB. Without proper precautions for this possibility, safety cannot be assured. It is even possible that minor leakage rate such as leakage class L0,01 (0,01 mg/m∙s) has such effect, in time the results can be devastating. Leakage could create short-circuits, corrosion of other components or loss of all electrolyte. Although the elastomer gaskets and sealing rings are minor costs in RFB, failure due to improper design and material selection bring unexpected costs if other components are damaged (figure 3). Developers of HBr redox flow battery work mostly with fluorinated elastomers (FPM) because of its universal chemical stability. Although leakage still frequently occurs after some time, especially in thin stacked designs it becomes a dreadful problem.

